
Western blot quantification using imagej how to#
It will also work a zone of background of the lane selected but if there are many bands (as you can see in the image) the peaks overlap (tha´t why I use a region quite far away). Quantification of foci using Fiji or ImageJ Protocol by Lee Pribyl, PhD, Cambridge, MA Updated 3/6/21 Purpose: This protocol describes how to quantify any type of cellular foci, such as phosphorylated Ser139 on histone variant H2A.X (H2AX) stained images of tissue or cells, using the free NIH Image Processing and Analysis in Java software called Fiji or ImageJ. That´s why I like to select an empty lane to give me an idea of the background (of course if the background is not even then you must repeat the blot). This tool provides a quick and dirty way to measure images of not necessarily straight lines of Western Blot films, dot blots and other silimar bio-scientific. 2) Press Ctrl +1 and drag the same rectangle selection to the next band. With this tool it is not possible to select regions between lane unless you left lanes unloades in the gel. 1) Open western image in Image J, select Rectangular Selections tool from the ImageJ toolbar and select first western band. I only show the first two lanes but you can see (in green) two peaks corresponding to the top band of the two first lanes, and the more intense band has clearly more area which at the end it is what ImageJ uses with the tool of gel analysis.
Western blot quantification using imagej manual#
But if you follow the ImageJ manual and use different regions for different lanes you can usually separate the peaks well enough. Even worse there is not peak in the first lane. As I show in the attached images if you open the Sample Gel in imageJ (Flie>Open Samples> Gel) when you select all the band of the same height in a single region the peaks are to close and it is imposible to separate then. Go to Analyze -> Gels -> Select first lane (then use ctrl + 2 to select all the other lanes and ctrl + 3 to plot the data). If necessary, scroll the image vertically by holding down the space bar and dragging. 7.For each peak, measure the size by clicking inside with the wand tool.
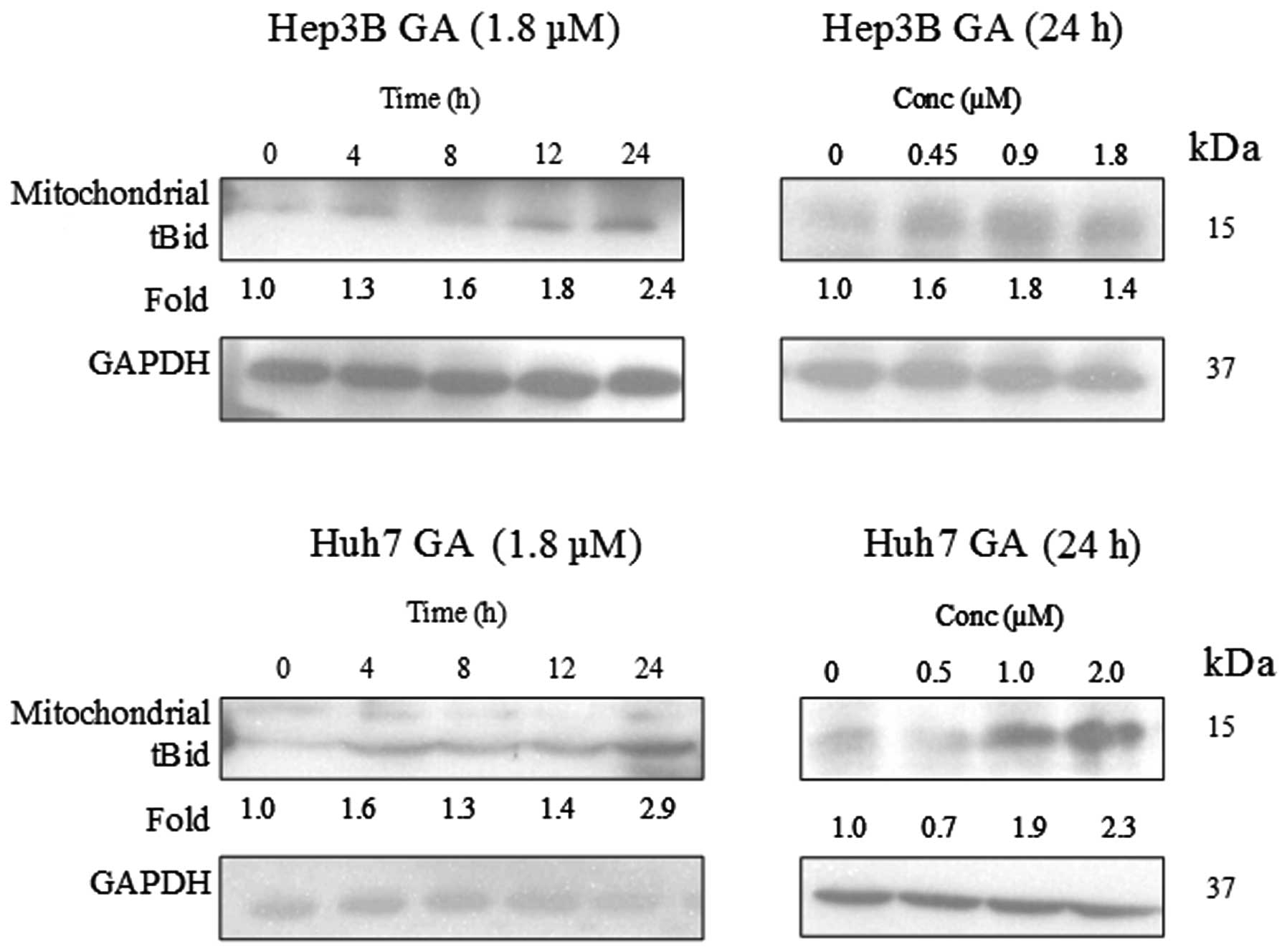
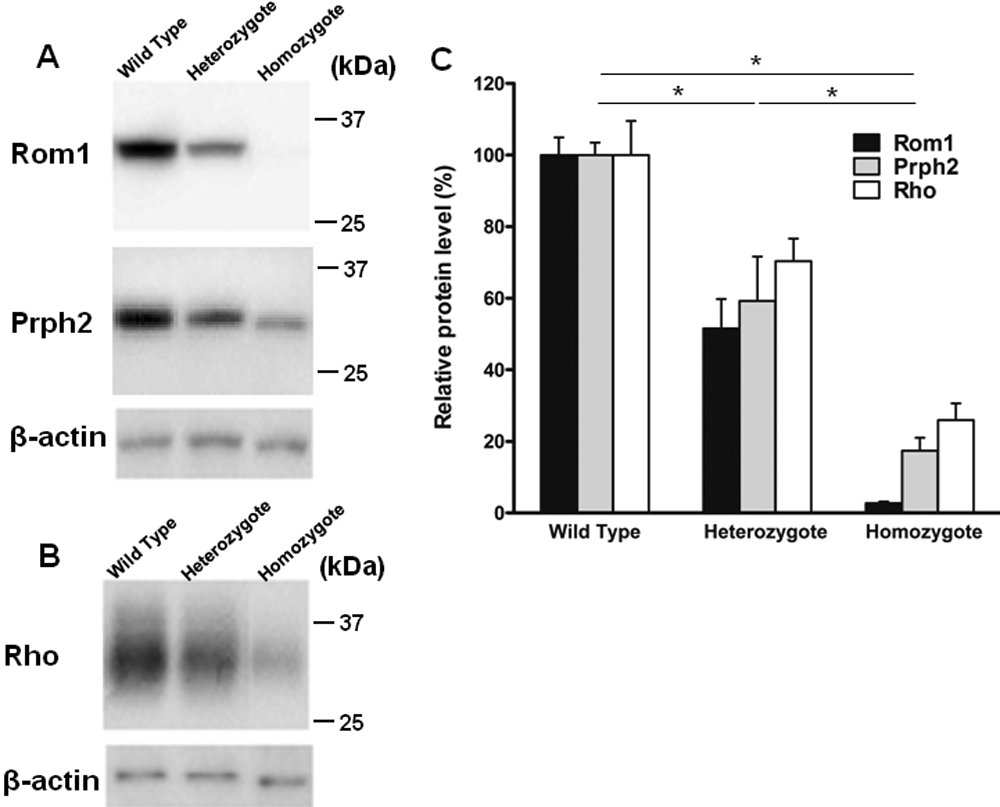
(Hold down the space bar to temporarily switch to this tool). On at least three fields of view per area of interest (tumor, normal lung tissue) (× 20 magnification), EGFR presence was quantified using ImageJ. To get to all the lanes, it may be necessary to scroll the image vertically using the 'Hand' tool. In surgical specimens, we separately analyzed tumor and normal lung tissue. But if Asa is analyzing WB with ImageJ he must select lane individualy and not use the same ROI for the same band in different lanes. ImageJ has a built in gel band quantification tool. For EGFR protein quantification, EGFR immunohistochemistry slides were digitized using a MIRAX Slide Scanner (3DHISTECH).


 0 kommentar(er)
0 kommentar(er)
